June is National Alzheimer’s & Brain Awareness Month!
The topic of Alzheimer’s can seem daunting, but this June we are excited to share that the FDA has made significant progress toward the pending approval of a new Alzheimer’s drug!
Key Announcement:
FDA Panel Vote: On June 10th, an expert panel organized by the FDA unanimously voted that Donanemab, a new Alzheimer’s medication developed by Eli Lilly, is effective and that the benefits outweigh the risks.
What is Donanemab?
Disease-Modifying Treatment: Unlike most Alzheimer’s medications that treat the symptoms, Donanemab targets the disease at its core, the brain. It introduces antibodies that bind to amyloid, a protein that has a toxic effect on neurons related to memory and cognitive function. These antibodies are designed to break down amyloid plaques, which slows the progression of Alzheimer’s disease.
What Makes This Treatment Different?
A Unique Approach: This trial is the first of its kind to study the stopping and starting of treatment, which is similar to the way cancer is treated with chemotherapy. Also similar to chemo, this medication is administered as a 30-minute infusion, once a month. Throughout this clinical trial, the company allowed some participants to stop treatment after their brain scans showed the amyloid plaque buildup had cleared.
Clinical Findings:
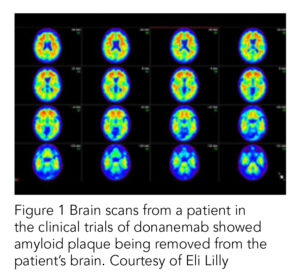
Effectiveness: These studies submitted by Lilly for the FDA review found that Donanemab slowed the progression of Alzheimer’s in patients at the earliest stages of their disease by 22% compared to those taking a placebo. It also reported a drop in amyloid plaques by over 80% compared to those receiving the placebo. The study found that Donanemab succeeded in clearing amyloid so much that the protein was no longer detectable on their brain scans.
Committee Conclusion:
When the study concluded, researchers determined that 60% of the participants could consider stopping their monthly infusions. This committee voted that for most patients with mild cognitive impairment, the drug effectively slowed their cognitive decline and the benefits of this medication outweighed the risks.
Ongoing Research:
While this trial has been deemed a success, researchers and the FDA still feel that there is more data to be collected before this treatment can be approved. It was concluded that more studies are needed in specific groupings of patients to determine the consistency of their findings.
Positive Outlook:

The 11-0 vote on the effectiveness of Donanemab is an encouraging sign for the ongoing research on this disease that affects so many of us. We are excited to share this news and will continue to keep our community abreast of findings on Alzheimer’s disease and its treatments.
Why Get an Early Diagnosis?
These findings support the importance of getting a diagnosis early. This drug, and many other Alzheimer’s medications, are consistently proven to be most effective when treatment starts in the early stages of memory loss.
Stay Informed:
For continued updates and support on Alzheimer’s, we encourage you to learn more about the Alzheimer’s At Home Navigator Program. Our team is here to aid and advise this growing New Canaan population.
We hope this progress brings hope and awareness to those affected by Alzheimer’s and their loved ones.
Warmly,
Rachel Takacs
Director of Navigation
203-594-5446

